Medicaid is the primary funding source for family planning services for low-income people and is jointly financed and administered by the federal and state governments. The federal Medicaid statute establishes minimum federal standards, and for decades, has classified family planning as a mandatory benefit category that all state programs must cover, but does not define exactly what services must be included. For the most part, these services are defined by the states within broad federal guidelines. This report presents findings from a 2021 survey of states on policies related to coverage of family planning services under Medicaid.
The range of family planning services that states make available to their beneficiaries is shaped by many factors, including longstanding federal policies related to coverage of family planning services, federal requirements for coverage of preventive services and prescription drugs, and states’ application of utilization controls such as maintaining preferred drug lists (PDL), requiring the use of generics before brand names, step therapy protocols, and prior authorization. States have considerable discretion regarding Medicaid eligibility criteria, managed care enrollment, and payment structures which also affect beneficiaries’ coverage for and access to family planning care as well as the amount, duration and scope of the services that are covered.
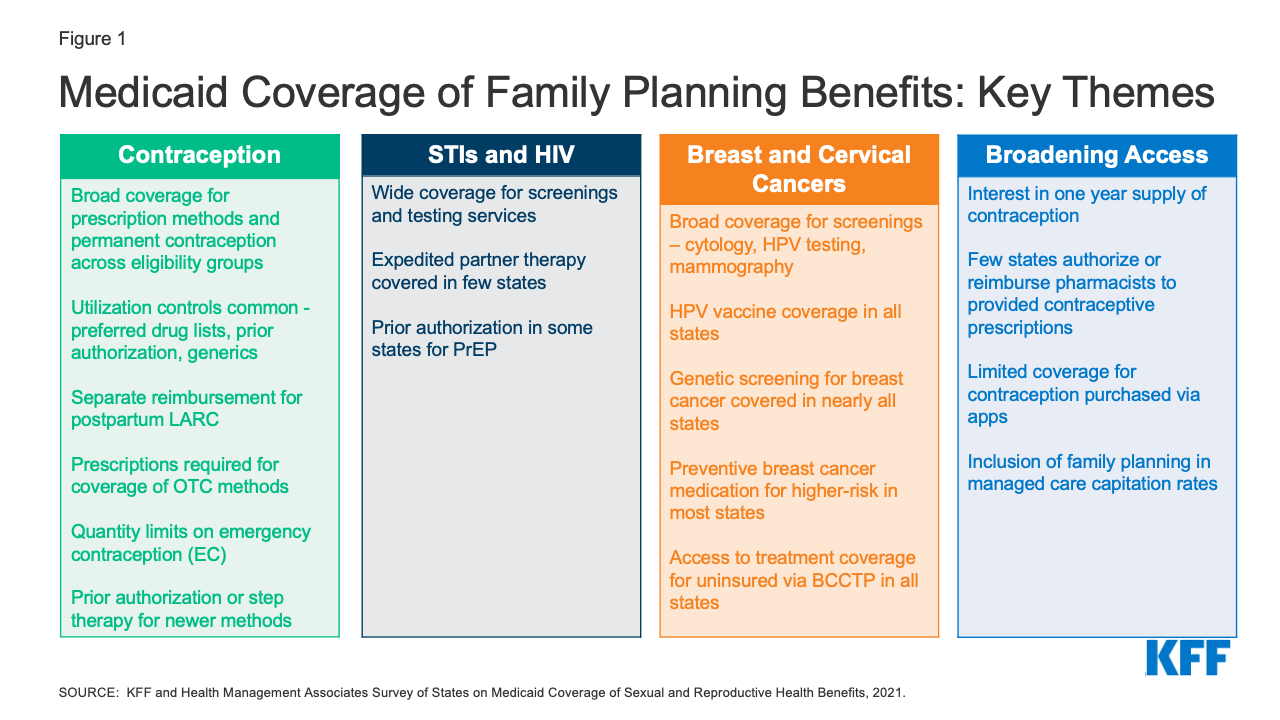
To obtain information about state Medicaid family planning coverage policies for adults, KFF and Health Management Associates (HMA) conducted a survey of state Medicaid agencies regarding coverage of sexual and reproductive health care services. Federal standards for different Medicaid eligibility pathways may vary: traditional Medicaid eligibility, which was in place prior to the Affordable Care Act (ACA), the Medicaid expansion pathway in states that have expanded eligibility under the ACA, and limited scope family planning programs for individuals who do not qualify through other pathways. Where relevant, differences in state policies between these pathways are highlighted. This report presents survey findings from the states that responded (41 states and District of Columbia) about coverage policies for fee-for-service Medicaid in place as of July 1, 2021, for the following categories of family planning benefits: prescription contraceptives, over-the-counter methods, STI and HIV services, well woman care, breast and cervical cancer services, and managed care services. Figure 1 summarizes key themes from the survey findings.

Figure 1: Medicaid Coverage of Family Planning Benefits: Key Themes
While all responding states (41 states and DC) cover prescription contraceptive methods approved by the Food and Drug Administration (FDA), many apply utilization controls such as quantity limits, age restrictions, generic requirements, and Preferred Drug Lists (PDLs). Federal rules require state Medicaid programs to cover all prescription drugs from manufacturers that have entered into a federal rebate agreement. As a result, all state Medicaid programs have open formularies that include coverage for all prescription contraceptives. However, to control costs and promote quality, states may employ utilization controls that can restrict access to specific drugs. Common controls include limiting the medication quantity that can be prescribed at one time, requiring use and trial of generics before a brand name product, implementing a preferred drug list, and requiring prior authorization before a certain product can be reimbursed. Some states, for example, use utilization controls to limit access to newer contraceptive products like the Annovera Ring and Phexxi.
Few states reported imposing utilization controls on coverage of intrauterine devices (IUDs) and implants. Most states also reported separate reimbursement for postpartum IUDs and implants rather than inclusion in a global payment for pregnancy-related services. IUDs and implants, the two forms of long-acting reversible contraceptives (LARCs), are among the most effective methods to prevent pregnancy and also the most expensive. In recent years, there have been considerable state and federal efforts to facilitate access to LARCs by improving reimbursement, particularly in the postpartum period, an important time for birth spacing and prevention of unwanted or mistimed pregnancies. Very few states reported imposing limitations on access to these methods, and most of the responding states reported reimbursing postpartum LARCs separately from a maternity global fee to clinicians and hospitals, averting what would otherwise result in a financial disincentive for postpartum LARC placement.
All responding states cover at least one form of emergency contraception (EC) pill under their traditional Medicaid program, but some states impose quantity limits and many require prescriptions for Plan B, even though it is approved for over-the-counter availability for EC pills. Emergency contraceptive pills prevent pregnancy if taken within the first few days after unprotected sex. They are not abortifacients as they cannot disrupt an established pregnancy. All but one state report coverage of prescription emergency contraceptive pills (ella or ulipristal acetate) across eligibility groups, and all but two cover over-the-counter (OTC) Plan B (levonorgestrel) under their traditional Medicaid programs. Far fewer states, however, reported covering Plan B without a prescription (7 states). Providing coverage without a prescription can expedite access, especially for a contraceptive with a short window of effectiveness such as emergency contraceptive pills.
Most states do not have a process for covering over-the-counter (OTC) methods such as condoms or sponges without a prescription. Thirty-eight states reported requiring a prescription from a provider to cover OTC methods, consistent with federal guidance that a prescription is required to obtain federal Medicaid matching funds. Ten states, however, reported covering some or all OTC contraceptives by expanding pharmacists’ scope of practice to prescribe and dispense specific contraceptives, either independently, under the supervision of a licensed provider with prescribing authority through a collaborative practice agreement (CPA), or through protocols such as a statewide “standing order.”
Nearly all reporting states cover testing and treatment for sexually transmitted infections (STIs) and routine HIV screening under their traditional Medicaid program, and almost all states align non-contraceptive family planning benefits across eligibility pathways within their state. Care for STIs is typically considered part of clinical family planning services. Under Medicaid, however, STI treatment is classified as a “family planning related” service. All responding states reported covering STI testing, treatment, and counseling under their traditional Medicaid program, and almost all align coverage across eligibility groups. Additionally, almost all the responding states also reported covering routine HIV screening in their traditional Medicaid programs.
Few states, however, reported covering Expedited Partner Therapy (EPT) which is endorsed by the CDC as an effective method to control the transmission of STIs. Expedited partner therapy (EPT) enables the treatment of the sexual partners of a patient diagnosed with an STI without examination and is recommended by the CDC for treatment of STIs. However, just nine of the responding states reported EPT coverage.
Some states require prior authorization for the provision of Pre-Exposure Prophylaxis (PrEP), a medication taken to prevent HIV acquisition, and some states do not cover it as a benefit under limited scope family planning programs. PrEP medications can prevent individuals from acquiring HIV, and are recommended for individuals at higher risk of HIV infection. Like other pharmaceuticals, Medicaid programs are required to cover PrEP, but 12 of the responding states reported having a prior authorization requirement. Seven states reported that they do not cover PrEP as part of their limited scope family planning programs, where coverage is optional because states can define the family planning and related services that they include for beneficiaries in these programs.
All the responding states cover services to prevent, detect, and diagnose cervical and breast cancer, but there is variation in the types of services that are included and whether they are covered under limited scope family planning programs. Screening for cervical and breast cancers is considered appropriate for provision during a family planning visit. Every responding state reported coverage in their traditional Medicaid program for HPV vaccines, cervical cancer screenings using cervical cytology and HPV tests, and colposcopy and LEEP or cold knife conization, which are recommended services following an abnormal screening. However, coverage for these services is not universal in limited scope family planning-specific programs.
All of the responding states cover screening mammograms for people eligible through the traditional Medicaid pathway, and most cover genetic screening (BRCA) and counseling as well as medication to prevent or reduce risk of breast cancer for women at higher risk. As with cervical cancer screenings, every participating state covers mammograms under traditional Medicaid, but not all cover them for enrollees in the limited scope family planning programs.
In addition to routine mammography, screening for genetic mutations and preventive medications are recommended for some women at higher risk for breast cancer. While these preventive services are considered optional under traditional Medicaid, 40 states cover genetic screening and counseling for BRCA mutations and 36 cover preventive medication for high-risk women in their traditional Medicaid program.
While nearly half of responding states cover a one-year supply of contraceptives at a time, few states allow pharmacists to prescribe and be reimbursed for contraceptive services provided to Medicaid beneficiaries. Extended supply and pharmacist prescribing of contraceptives are two avenues for enhancing access to family planning services. A number of states report that they allow Medicaid coverage for a one-year supply of certain hormonal methods, including 18 states that permit a one-year supply of oral contraceptives. However, fewer than a dozen of the responding states reimburse for pharmacist provision of contraceptives.
The availability of contraceptives via online apps is proliferating, but few states provide Medicaid coverage of contraceptives obtained through these platforms. In recent years, a number of companies have been providing mostly hormonal contraceptive methods through online platforms for customers to obtain contraceptives typically prescribed using an asynchronous telehealth protocol. While some of these companies do not accept any third-party payments, eight states reported that Medicaid covers contraceptive purchases secured through these apps. This is an evolving area, but overall Medicaid coverage for these products is limited at this time.
Medicaid represents a significant source of coverage of the full range of contraceptive methods and related family planning services for low-income people. In recent years, Medicaid enrollment, particularly of reproductive age adults, has grown as a result of state decisions to expand Medicaid under the ACA and to establish limited scope family planning programs. This survey finds robust coverage of many contraceptive services and supplies, but variation in the application of utilization controls. While there is broad coverage for prescription contraceptives (due to requirements and the drug rebate program) access to newer and OTC methods as well as adoption of policies that have been demonstrated to facilitate access, such as 12-month dispensing or allowing pharmacists to prescribe and be reimbursed, are less common. Furthermore, some states have not adopted protocols that facilitate the prevention of STIs and HIV such as expedited partner therapy or coverage for PrEP without prior authorization, important public health advances that have the potential to improve the sexual health of high-risk populations. In the coming years, particularly if access to abortion services becomes increasingly limited, the choices that states make regarding Medicaid eligibility and coverage for family planning services will make a critical difference in the reproductive health and well-being of millions of people across the nation.
Medicaid, the nation’s health coverage program for low-income people, plays a primary role in financing and providing access to sexual and reproductive health services for millions of low-income individuals. The program covers more than 20 million adults ages 18 to 49 1 and is the largest source of public funding for family planning services. The program is operated jointly by the federal and state governments, who share responsibility for payment of services, while states set eligibility levels and determine the amount, duration, and scope of covered benefits within broad federal parameters.
Financing and coverage of family planning services is unique within the Medicaid program. Federal Medicaid law classifies family planning services and supplies as a “mandatory” benefit category that states must cover, but it does not formally define the specific services that must be included, giving states discretion as to which services they include in this category. In addition, federal law:
Coverage for prescription drugs is another important element in Medicaid coverage of family planning services. All states have chosen to cover prescription drugs, even though it is an optional benefits category under federal law. Furthermore, all state Medicaid programs must maintain an “open formulary,” meaning that Medicaid covers nearly all FDA-approved drugs from manufacturers that agree to provide rebates for a portion of drug payments. States, however, can impose utilization control policies to limit spending and promote quality, which can restrict access to some drugs, including certain contraceptives.
Enrollees who qualify for Medicaid through traditional pathways, those in place prior to the Affordable Care Act (ACA) are entitled to coverage for family planning services. Several states have established special limited scope “family planning programs” that extend Medicaid coverage for family planning services only to individuals who are not eligible for traditional Medicaid (usually because their incomes exceed the state income eligibility thresholds or do not otherwise qualify for Medicaid). States can establish family planning-only programs either through federal Section 1115 research and demonstration waivers or State Plan Amendments (SPAs) that must be approved by the Centers for Medicare and Medicaid Services (CMS), the federal agency that oversees the Medicaid program. States can decide which services they cover in these limited scope family planning programs, and pharmacy coverage under limited scope family planning programs is restricted to family planning and related services.
Additionally, states that have opted to expand Medicaid eligibility under the ACA are required to cover “essential health benefits,” including preventive services recommended by the U.S. Preventive Services Task Force (USPSTF), preventive services for women identified by the federal Health Services and Resources Administration (HRSA) based on the recommendations of the Women’s Preventive Services Initiative (WPSI), and vaccines recommended by the Advisory Committee on Immunization Practices (ACIP). The slate of preventive services recommended by these committees include several family planning and related services, specifically FDA-approved, authorized and cleared contraceptives with a prescription, screenings for STIs and HIV, screening for cervical and breast cancers, the HPV vaccine, well woman visits, and screening for intimate partner violence. These services must be covered under ACA Medicaid expansion, but that requirement does not apply to traditional Medicaid or limited scope family planning programs, which means that the benefits package could vary within a state for different Medicaid populations (Table 1). However, a 2015 KFF/HMA survey found that most states have aligned coverage of family planning benefits for all pathways, despite the differing requirements. States do vary, however, in the utilization controls that they choose to apply.
To understand the scope of coverage for sexual and reproductive health services, the utilization controls that states adopt, variations between and within states, and related state Medicaid policies across the nation, KFF (Kaiser Family Foundation) and Health Management Associates (HMA) conducted a national survey of states about policies in place as of July 1, 2021. States were asked primarily about coverage of services under traditional Medicaid and whether they align coverage policies in limited scope family planning programs and under their Medicaid expansions, where applicable.
The survey was conducted between June 2021 and October 2021. Forty-one states and the District of Columbia responded to the survey (Figure 2). As of July 1, 2021, 31 of these participating states had implemented the ACA Medicaid expansion, and 11 had not implemented the expansion. Since July 1 st , one of the 11 non-expansion states (Missouri) has implemented Medicaid expansion. Of the responding states, 24 states also offer limited scope family planning programs financed by Medicaid to individuals who do not qualify through other Medicaid pathways. Two additional states (Iowa and Missouri) operate limited scope family planning programs that are entirely state-funded because they exclude providers that offer both family planning and abortion services, disqualifying those programs from federal Medicaid payments. These state restrictions violate Medicaid’s freedom of choice requirement and Medicaid’s requirement to include all willing providers, which give Medicaid beneficiaries the right to seek services from any qualified provider that participates in a state’s Medicaid program. States that did not respond to the survey are: Arkansas, Georgia, Kentucky, Minnesota, Nebraska, New Hampshire, New Mexico, Ohio, and South Dakota.
Presented below are detailed survey findings from 41 states and DC concerning coverage and utilization limits for reversible contraceptives and permanent contraception, well woman care, STI and HIV services, services for breast and cervical cancers, and requirements for managed care plans regarding coverage of family planning services. A majority of the states responding to the survey contract with managed care organizations (MCOs) under a capitated structure to deliver Medicaid services, including family planning. While the survey’s questions focused on state Medicaid policies and coverage under fee-for-service, these policies form the basis of coverage for MCOs.